
Avansee Preload1P is designed with the surgeon in mind
All Avansee lenses are manufactured from hydrophobic, highly cross‑linked, soft acrylic. There have been no reports of glistening since their launch in 2007.1–3
IOLs were incubated in saline at 45 ± 1°C for 24 hours and then at 37 ± 1°C for a further 2.5 hours to allow glistening to form.4

CP2.2R+26.0D

SN60WF+20.0D
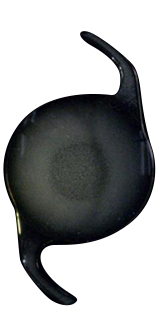
NY60+20.0D
Low PCO rate
The smooth lens surface and 360º square edge design, which extends around the lens including the optic-haptic junction6, are associated with reduced posterior capsule opacification (PCO) formation.7,8
Unique haptics maintain space between optic and haptic, aiding adhesion between the anterior and posterior capsule, preventing PCO.8
Hydrophobic IOLs are associated with reduced risk of PCO, compared to hydrophilic IOLs.9
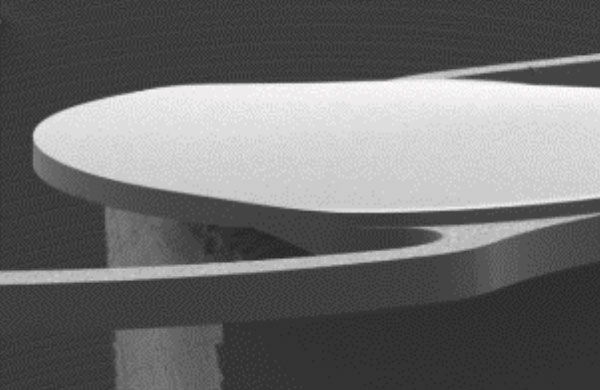
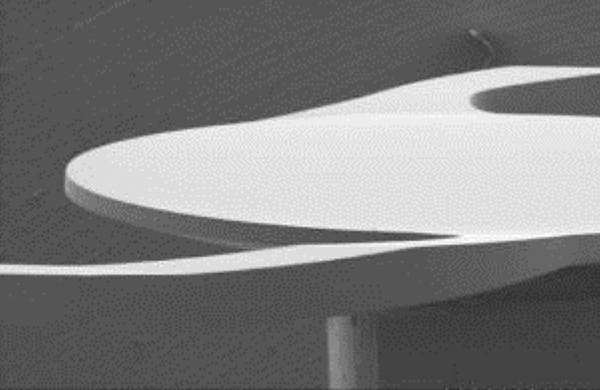
Configuration of the 1P lens10
360° square edge for all lenses3,7,8
Posterior optic
x100




Excellent vision quality
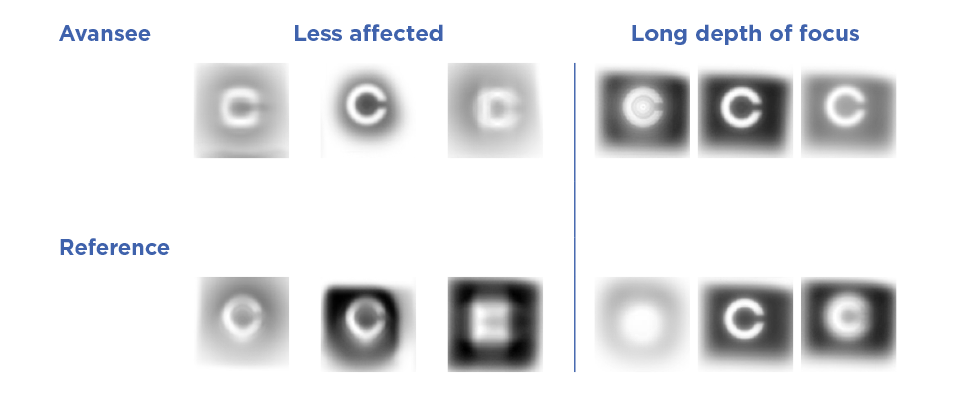
The low asphericity of Avansee lenses means they provide a long depth of focus and are less influenced by IOL misalignment (decentration and tilt).9,11
IOLs that have little effect on the spherical aberration of the eye can potentially improve the quality of vision by reducing the risk of coma aberration associated with IOL misalignment (decentration and tilt).
The asphericity of Avansee is -0.04μm.11
SIMULATION USING A MODEL EYE (SIMULATION CREATED BY THE OPTICAL DESIGN SOFTWARE (ZEMAX) WITH THE FOCAL POINT MADE AT 3 METRES)12,13
Proven long term safety
Over 1 million Avansee lenses sold since launch in 2007, with very low incidences of adverse events (0.009%) and serious adverse events (0.0009%).15,16
